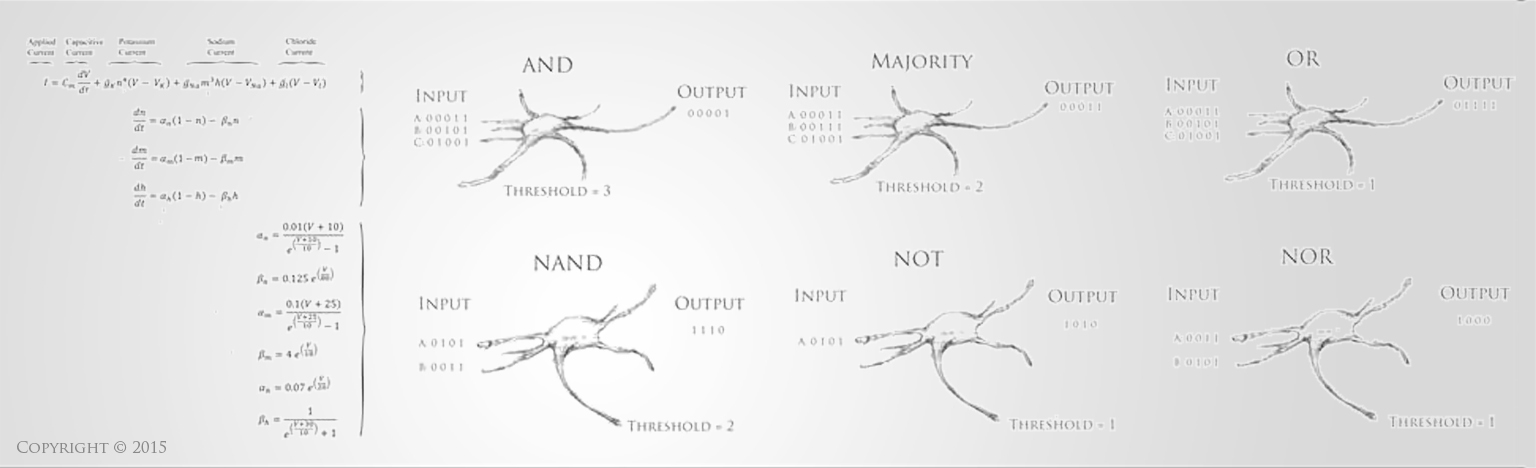
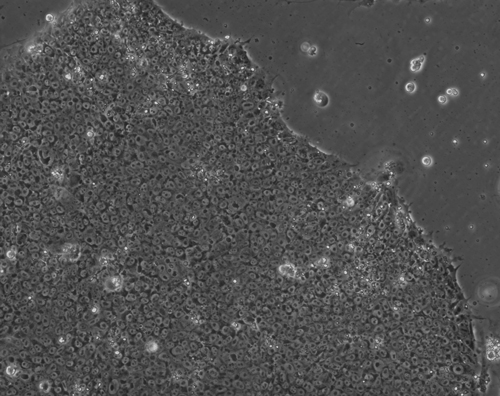
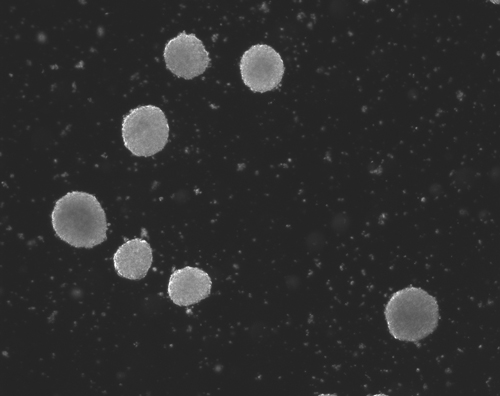
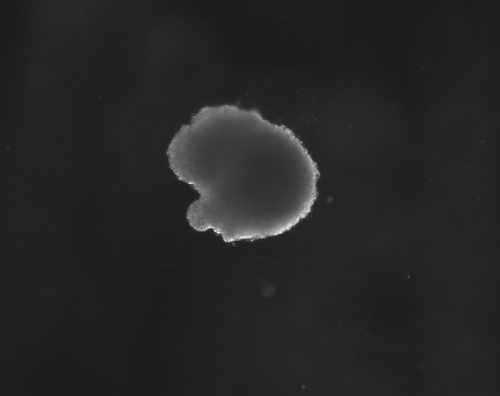
Stem Cells hold incredible potential to heal and regenerate tissues due to the fact that they retain capabilities to become a variety of more specific types of functional cells and tissues. For neural tissue engineering, the goal is not only to restore neurons, but also the framework of neural networks and the essential neural-supportive astroglial cells. Guiding cells to achieve the intended goal of survival, proliferation, differentiation, and network formation is a difficult challenge, but many recent promising advancements have placed this goal within our grasp. The Institute has optimized techniques for deriving 3D neuronal cells from iPSCs of patients' skin cells. The process involves several steps, including reprogramming and passaging of pluripotent stem cells, formation of spherical aggregates of stem cells, induction of 3D neurospheres, and the formation of differentiated neuronal subtypes in 3D tissue organoids (Figure 1) [1].
Figure 1(a-d): Perhaps resembling the astronomical creations of the universe suspended in the voids of empty space, these images show the wondrous process of human neural tissue differentiating from stem cells: (a) Thousands of induced pluripotent stem cells on a 2D surface, (b) Spherical aggregates of stem cells, (c) 3D neurospheres, (d) 3D differentiated neural tissue showing neuroepithelial formation and early neural differentiation with many thin neurite processes extending from the organoid. [Images by Dr. Richard McMurtrey]
   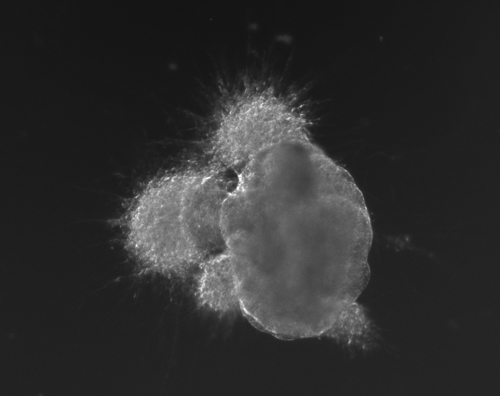
The concept and existence of stem cells was first recognized by Alexander Friedenstein, a Soviet scientist working on potential treatments for people exposed to high radiation doses at a time when nuclear exposure was at the forefront of many people’s minds. Following the earlier work of Alexander Maximov, a Soviet scientist and physician who in 1909 had proposed that all blood cells had a common lineage from a “Stammzelle,” or “stem cell” [2], Friedenstein found that unique cells could proliferate into a variety of blood cell types [3-4]. Many characteristics of stem cells were later described by yet another Soviet scientist and physician, Joseph Chertkov, who elucidated that different types of stem cells could exist and that they could be affected in their differentiation by their local environment and position within tissue [5].
Many types of cells hold potential for beneficial use in neural regeneration, including embryonic stem cells (ESC), induced pluripotent stem cells (iPSC), endogenous stem cells, or possibly even more mature cell types, but each of these has certain advantages and disadvantages, which are only briefly discussed here. Embryonic stem cells retain the ability to become any type of cell in the body, but must be dissociated from an embryo in its early stages, raising many ethical concerns. Fortunately, iPSCs can also become any cell in the body and they may be induced from a variety of cell types (such as skin or blood) to become neuroglial progenitor cells, meaning that a patient may provide stem cells from his or her own body. The ability to produce iPSCs was first demonstrated in 2006 with mice fibroblasts [6], and a year later with human fibroblasts [7]. It has now been shown that human iPSCs can be made into a variety of neural cells capable of forming functional synapses [8], thus demonstrating much promise for future approaches in neural tissue engineering to cure injuries and diseases like stroke, spinal cord injury, traumatic brain injury, movement disorders, and neurodegenerative diseases [9-10].
In 2014, for the first time, iPSCs were implanted into the same patient who donated the cells. In this procedure, skin cells were taken from an elderly woman who suffered from macular degeneration, and these skin cells were converted into iPSCs and induced into retinal pigment epithelium cells, which were implanted as a small 2-dimensional sheet onto the retina. This work was carried out by world-recognized leaders in stem cell research in Japan, and although it is unlikely to restore vision, as the first procedure of its kind it will help to demonstrate safety and feasibility [11-12]. The ability to create neurons from skin cells not only creates incredible potential for neural regeneration therapy, but also enables the capability to investigate pathological conditions of the nervous system. Using iPSCs derived from patients with neurological diseases, researchers can create and study these diseased human cells under controlled conditions as "cerebral organoids" and thereby derive new understanding and treatments that might otherwise not be possible. Study on such induced neural cells has already begun for Parkinson’s disease and other neurogenetic syndromes [13-18]. Evidence suggests that stem cells may also be capable of not just reconstructing neural architecture, but also providing many trophic factors that promote cell survival and protection [19-25].
The source of cells is thus an important question that must be considered carefully, and the desired cell type determines the options for cell retrieval. The optimal choice of cell type will depend on how effectively the cell can survive, differentiate, function, and integrate into an operational state within the tissue, as well as on the ease of retrieval and use in a patient. It must also be decided whether to retrieve the cells directly from the intended patient (autologous grafting), or from one patient for use in another patient (allogenic grafting). The advantages of autologous grafting are that, at least in theory, the risks of immunorejection of the transplanted cells are greatly decreased, and studies also suggest that stem cells may be more immunocompatible than previously thought [26-27]. However, in patients with genetic diseases, autografting cells perpetuates the same genetic problems, although it is hypothesized that in multifactorial neurodegenerative diseases the reversion to a stem cell state can reset the clock for the disorder and also provide new supportive cells that augment neuronal survival and function. Furthermore, the ability to correct mutated genes in harvested cells is becoming a real possibility for many disorders.
It has been observed that differentiation into neural stem cells mirrors the development process and stages that occur in real life [8,28]. This emphasizes the importance of understanding embryological processes in the development of neural tissue in the early stages, and will likely hint at ways that this developmental patterning can be replicated. These neurodevelopmental processes involve a complex array of biological and physical signals that guide and shape neural structure and function, and it is quite plausible that neural regeneration can best be achieved by attempting to mimic molecular cues that exist during embryologic development and by patterning targeted neuroanatomical structures. The combined roles of stem cell biology, molecular signaling, structural mechanics, and material engineering hold much potential to expand our understanding and achieve success in this work [9-10]. Additional information on stem cells for patients is provided by the International Society for Stem Cell Research. Some clinical studies and therapeutic interventions are currently available at Alpine Spine & Orthopedics Institute. Translating this research into effective clinical therapies will require significant additional research and resources, which are simply not achievable without the support of many generous and interested donors. If you would like to contribute to these ground-breaking efforts, please consider donating today for tomorrow's discoveries.
-------------------
References:
1) McMurtrey RJ. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Engineering Part C. 2015.
2) Maximow A. Der Lymphozyt als gemeinsame Stammzelle der verschiedenen Blutelemente in der embryonalen Entwicklung und im postfetalen Leben der Säugetiere. Folia Haematologica 8.1909, 125-134. Republished in: Cell Ther Transplant. 2009,1:e.000040.01.
3) Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83-92.
4) Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976 Sep;4(5):267-74.
5) Chertkov JL, Gurevitch OA. Hematopoietic stem cell and its microenvironment. Moscow. Meditzina, 1984.
6) Takahashi K, Yamanaka S. Cell. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. 2006 Aug 25;126(4):663-76.
7) Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861-72.
8) Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012 Feb 5;15(3):477-86, S1.
9) McMurtrey RJ. Patterned and Functionalized Nanofiber Scaffolds in 3-Dimensional Hydrogel Constructs Enhance Neurite Outgrowth and Directional Control. J. Neural Eng. 11 (2014) 066009
10) McMurtrey RJ. Novel Advancements in Three-Dimensional Neural Tissue Engineering and Regenerative Medicine. Neural Regeneration Research. 2015 Mar; 10(3).
11) Kanemura H, Go MJ, Shikamura M, Nishishita N, Sakai N, Kamao H, Mandai M, Morinaga C, Takahashi M, Kawamata S. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One. 2014 Jan 14;9(1):e85336.
12) Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014 Jan 23;2(2):205-18. [Also see http://www.nature.com/news/japanese-woman-is-first-recipient-of-next-generation-stem-cells-1.15915]
13) Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schüle B, Dolmetsch RE, Langston W, Palmer TD, Pera RR. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011 Mar 4;8(3):267-80.
14) Paşca SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, Paşca AM, Cord B, Palmer TD, Chikahisa S, Nishino S, Bernstein JA, Hallmayer J, Geschwind DH, Dolmetsch RE. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011 Nov 27;17(12):1657-62.
15) Krey JF, Paşca SP, Shcheglovitov A, Yazawa M, Schwemberger R, Rasmusson R, Dolmetsch RE. Timothy syndrome is associated with activity-dependent dendritic retraction in rodent and human neurons. Nat Neurosci. 2013 Feb;16(2):201-9.
16) Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, Krawisz A, Froehlich W, Bernstein JA, Hallmayer JF, Dolmetsch RE. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013 Nov 14;503(7475):267-71.
17) Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng PY, Klyachko VA, Nerbonne JM, Yoo AS. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. Oct. 22, 2014.
18) Okano H, Yamanaka S. iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain. 2014 Mar 31;7:22.
19) Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013; 2013: 786475.
20) Martinez AM, Goulart Cde O, Ramalho Bdos S, Oliveira JT, Almeida FM. Neurotrauma and mesenchymal stem cells treatment: From experimental studies to clinical trials. World J Stem Cells. 2014 Apr 26;6(2):179-94.
21) Ribeiro-Resende VT, Pimentel-Coelho PM, Mesentier-Louro LA, Mendez RM, Mello-Silva JP, Cabral-da Silva MC, de Mello FG, de Melo Reis RA, Mendez-Otero R. Trophic activity derived from bone marrow mononuclear cells increases peripheral nerve regeneration by acting on both neuronal and glial cell populations. Neuroscience. 2009 Mar 17;159(2):540-9.
22) Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010 Jan;27(1):131-8.
23) Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004 Jan;21(1):33-9.
24) Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13594-9.
25) Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao C, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol Cell. 2014 Oct 23;56(2):193-204.
26) Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011 May 13;474(7350):212-5.
27) Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H, Fairchild PJ. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20920-5.
28) Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002 May;5(5):438-45.
-------------------
Copyright © 2014 Institute of Neural Regeneration & Tissue Engineering. All rights reserved on all text and images, no use allowed without written permission and proper attribution and acknowledgment of source.
Back to Education |